Research Professionals is a leading GCP compliant Contract Research Organization (CRO) based in Hungary with operations in Poland, Czechia, Romania, Bulgaria and Serbia, serving customers from across Europe and the globe.
Research Professionals CRO (RP-CRO) is dedicated to providing the most efficient, flexible, and high quality Good Clinical Practices (GCP) compliant clinical research management services to CRO customers. RP-CRO leverages its extensive Central and Eastern European (CEE) network of highly experienced Principal Investigators (PIs) with their large local subject pools that can accelerate study enrolment for a wide range of clinical research study types. Our CRO team focusses on efficiently driving your clinical research study to completion and preparing the highest-quality submissions for leading regulatory bodies including the U.S. FDA and EMA.
Global Alliance of CROs
Research Professionals (RP) is the founder of ACROSS Global™, a strategic hub of CROs. We can cover 95+ countries, providing access to 7,500+ study sites, and plenty of potential clinical trial subjects.
HR search for clinical research
We ensure the fulfillment of our staff with a unique skill development tool. We help them in achieving goals and we support their personalized growth – step by step.
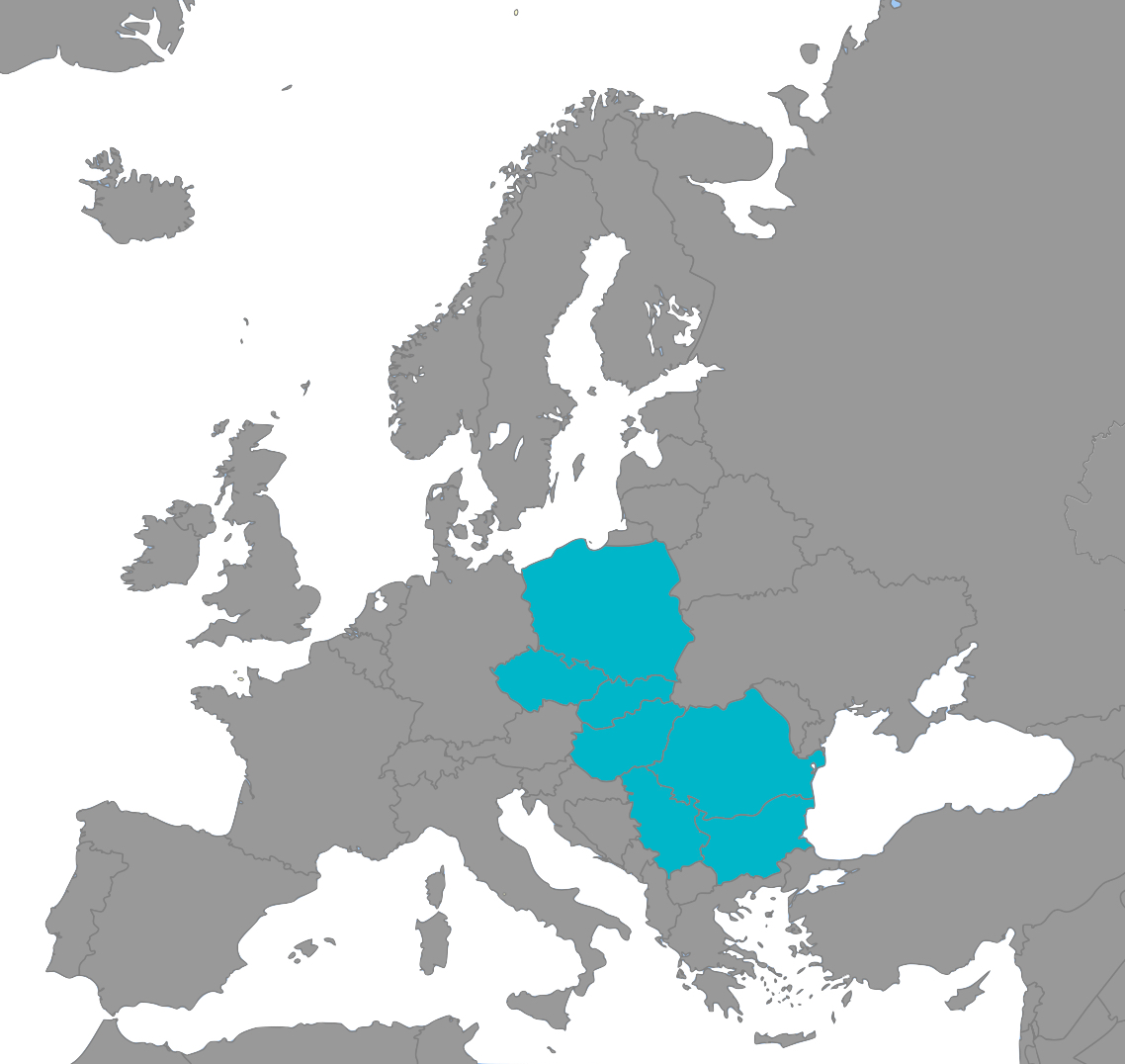
Regional Coverage

HR search for
clinical research
Global Alliance of CROs

Regional Coverage

HR search for clinical research

Global Alliance of CROs

Regional Coverage
Putting the C in CRO
Clinical
We are experts in managing all phases of clinical research studies for a wide range of pharmaceutical, device, and surgical test articles. With clinical operations in Hungary, Poland, Czechia, Romania, and Bulgaria, RP-CRO can efficiently support your clinical study requirements no matter what they are. We are also leaders in the management of Decentralized Clinical Trials (DCTs) that bring the clinic to the study subjects.
Capable
RP-CRO operates clinical facilities and employs staff with the capabilities to efficiently deliver world class CRO services. We assign our flexible in-house clinical teams to customer studies when and where they need them. This allows us to complete clinical research studies faster, meeting aggressive timelines without sacrificing quality.
Compliant
RP-CRO has built robust quality systems that ensure we strictly follow GCP regulations and use a CenterWatch based Quality Management Systems (QMS) resulting in high-quality clinical trial submissions for global customers.
Cost-efficient
Headquartered in Central and Eastern Europe (CEE) in a European Union (EU) Member state, RP-CRO is able to deliver clinical research management services to western market quality standards for less. We leverage accelerated enrolment from large available local research subject pools, use leading Principal Investigators (PIs) and our own flexible staff to reduce study timelines and overall program costs.
Compassionate
Our clinical research management staff care about our study subjects first and foremost. This ultimately benefits the patients who can access the new medicines and medical devices that successfully emerge from these clinical research studies. For RP-CRO, taking care of the subjects in our studies means safe and compliant facilities, highly qualified medical staff and paying attention to the details. Our subjects matter.
We Are Research Professionals – Your Next CRO
We are called Research Professionals because that is what we always strive to be. Our clinical research teams are entirely dedicated to providing customer focused clinical research management services that make a difference in patient’s lives. As a full service CRO, we provide complete clinical research study management services that are designed to deliver quality from an organization built on flexible efficiency. At RP-CRO, our track record of successfully completing clinical studies to the tightest timelines is only possible because of the exceptional abilities of our highly trained research professional staff and the comprehensive streamlined procedures they follow to ensure consistent quality outcomes. Engage RP-CRO on your next clinical research study to find out why our many international customers prefer to go with the professionals at Research Professionals CRO.
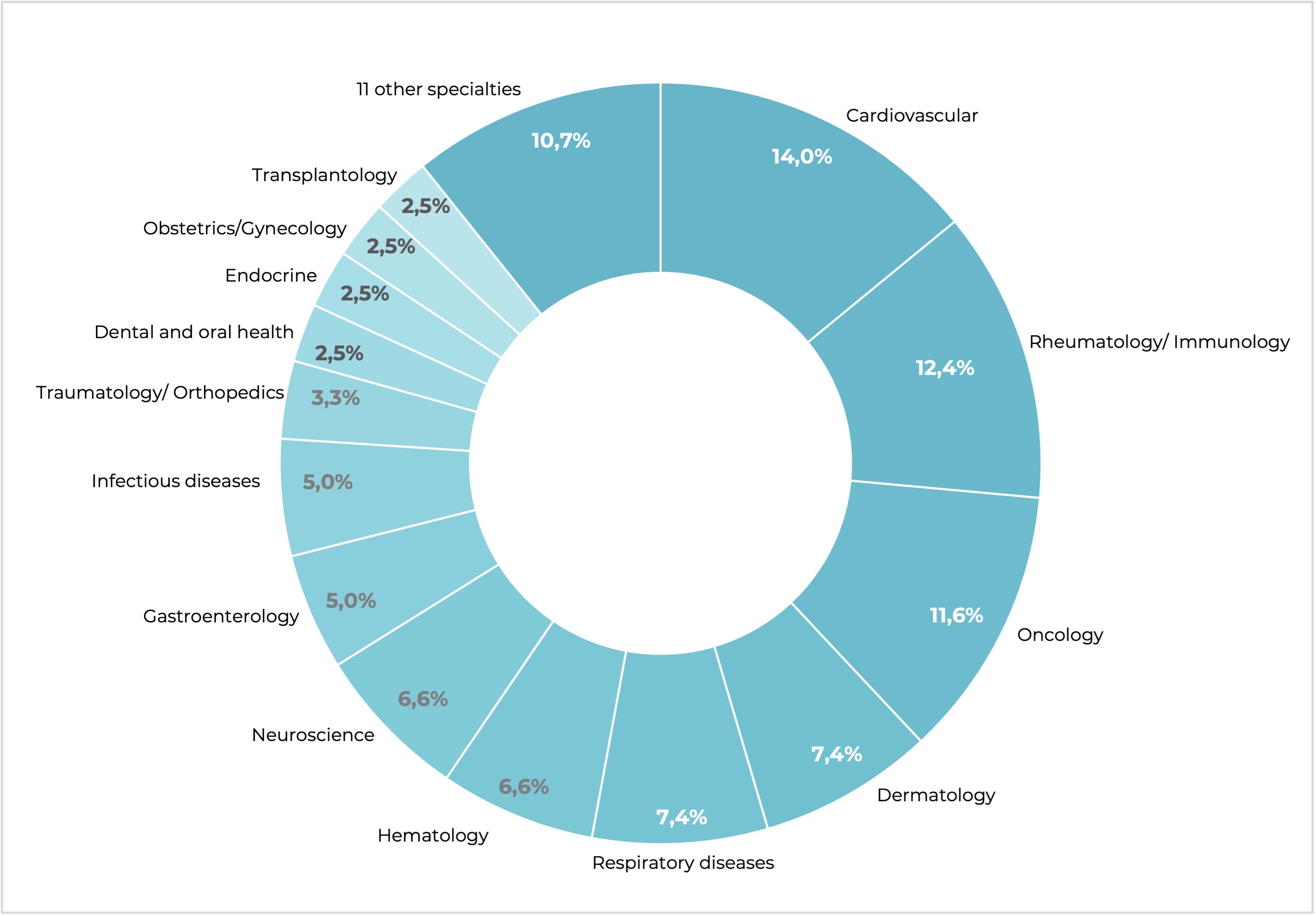
Facts & Figures