Clinical Study Quality with Central and Eastern European (CEE)-based Contract Clinical Research Organizations (CROs)
November 2024
Dr Zoltan SAGI, Business Development Director, Research Professionals CRO
Introduction
Based on recent U.S. Food and Drug Administration (U.S. FDA) data compiled over the last 15 years, clinical studies conducted in Central and Eastern European (CEE) countries have earned some of the highest frequencies of No Action Indicated (NAI) findings by the agency. This is the best possible inspection result indicating that no objectionable conditions or practices were observed during the inspection. Correspondingly, CEE based clinical sites also had some of the lowest percentage of inspections that resulted in Voluntary Action Indicated (VAI) and most importantly Official Action Indicated (OAI) status, even when compared to inspections of North American and Western European sites. VAI means that objectionable conditions or practices were found but that the U.S. FDA does not recommend or require any administrative or regulatory actions be taken. OAI findings will require the sites to comply with administrative or regulatory actions mandated by the agency. Inspections with OAI findings may indicate serious quality or compliance issues that must be remedied in order to regain compliant status.
So how did CEE based clinical sites achieve such positive results? This paper will focus on Contract Clinical Research Organizations (CROs) and clinical research sites located in CEE countries that are also part of the European Union. It will analyze the potential sources of the strong quality indicators described in the inspections as well as examine the numerous contributors to these documented inspection-based findings. The paper will also identify the actions and factors at the country, site, and CRO levels that contribute to the ability of CEE-based clinical trials to reliably deliver high-quality study results that meet or exceed both the sponsor organization’s and the regulatory body’s expectations. CRO operational parameters that can be studied for quality impact include deviation management, audit results, inspections, product advancement and marketing approvals. Quality must be all encompassing for the Clinical Research Organization (CRO), from start to finish, in order to maintain their customer’s critical timelines, adhere to budget and generate high-quality Good Clinical Practice (GCP) compliant data that will be accepted by leading regulators around the world. A CRO with well-trained and educated staff, robust standard operating procedures (SOPs), and efficient, transparent operational practices can minimize the risk associated with study protocol design, data management, scientific review, Principal Investigator (PI) qualification and clinical study personnel. A forward thinking CRO, especially those based in CEE countries, have a proven track record of delivering high quality studies through their dedication to rigorously following GCP principles. This paper will delve into the many factors that contribute to the clearly positive results we see reflected in U.S. FDA inspections of CEE sites over the last 15-year period.
About Research Professionals
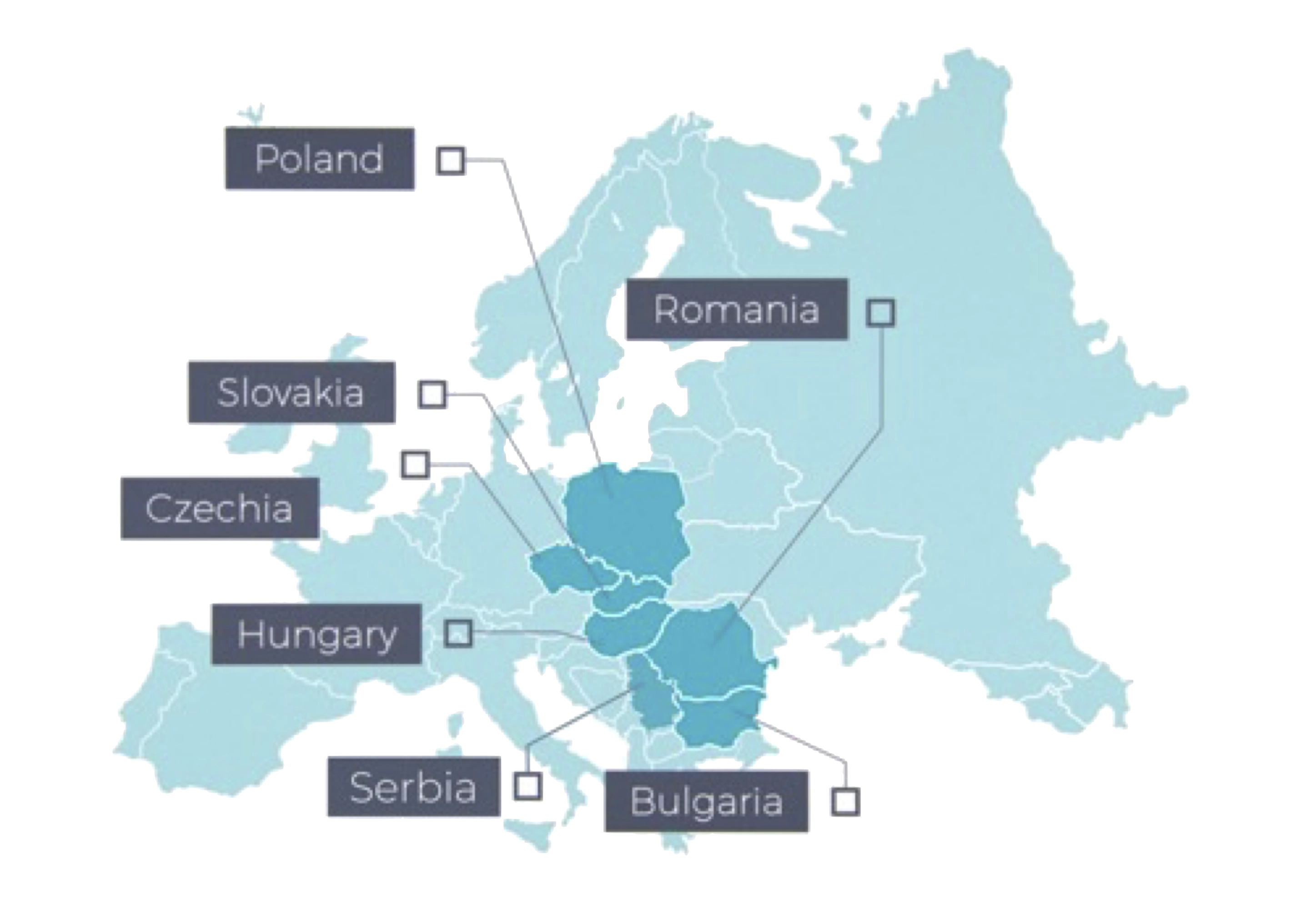
Research Professionals (RP-CRO) is a leading GCP compliant CRO based in EU member state Hungary with operations in Poland, Czech Republic, Slovakia, Romania, Bulgaria, and Serbia, serving customers from across Europe and around the globe. RP-CRO has developed leading clinical study expertise, drawing upon an established base of highly qualified Principal Investigators (PIs) with access to large study participant pools and modern clinical infrastructure that can accelerate study enrollment. With more than 150 experienced and dedicated staff, RP-CRO brings proven experience gained from managing over 75 studies in more than 15 therapeutic areas, both for drugs and medical devices, to every sponsor study, large or small. As Research Professionals’ operations are predominantly located in EU member countries, they are subject to EMA regulations and quality standards, ensuring that its services meet the strict quality standards of the world’s leading clinical research markets. RP-CRO’s non-EU Serbian operations provide a flexible and efficient research service option, that can help meet aggressive timeline and budget requirements. Research Professionals – DCT is a business division that specializes in conducting decentralized clinical trials (DCTs) bringing the study to the patient across all of Europe.
Want to learn more?
Author Biography
Dr Zoltán SÁGI, Business Development Director, Research Professionals CRO
Dr Sági is a respiratory physician with an economist degree in marketing. He has broad experience in strategy and business planning, partnership development, investor search and deal-flow management. Proven success in mentoring teams for desired outcomes achievement and ensuring tactical alignment with medical needs. Well-versed in portfolio management, medical risk analysis, clinical performance study, effective solutions, and regulatory compliance. Expert at directing and executing medical operations, managing resources, demonstrating scientific acumen, and transforming clinical data into value propositions. Possessed collaboration, communication, decision-making, and presentation skills, operating well in matrix environment.
References:
- U.S. FDA Inspections Data 2009-2023 (https://datadashboard.fda.gov/ora/cd/inspections.htm)
- Decentralized Clinical Trials: Are we ready to make the leap? January 29, 2019, BiopharmaDive, https://www.biopharmadive.com/spons/decentralized-clinical-trials-are-we-ready-to-make-theleap/546591/#:~:text=Recruiting%20and%20retaining%20patients%20for,Patient%20Innovation%20Center%20at%20PAREXEL
- Clinical Trials in Central & Eastern Europe: Keeping Up with Global Competition, Journal for Clinical Trials, Volume 9, Issue 2, 2017, https://journalforclinicalstudies.com/wp-content/uploads/2017/04/Clinical-Trial-in-Central-and-Eastern-Europe.pdf
- Pharma Intelligence Center, Global Data, extracted May 31, 2024, monthly enrollment by number of sites and trial count
- EU Clinical Trials Register, for period January 1, 2014 to December 31, 2023, all trials by phase and country https://www.clinicaltrialsregister.eu/ctr-search/search?query=&country=si&phase=phase-three&dateFrom=2014-01-01&dateTo=2023-12-31
- Tatyana Benisheva, Dimitar Milkov, Valentin Kopanarov, Ivaylo Ivanov, Dimitar Dimitrov, Veselina Todorova, Nigyar Dzhafer, Iva Chavkova, Lyubina Todorova & Lena Gebert (2023) Conducting clinical trials in five Eastern European countries (EU-EECs) with a focus on Bulgaria, Biotechnology & Biotechnological Equipment, 37:1, 2226741, DOI: 10.1080/13102818.2023.2226741 https://www.tandfonline.com/doi/full/10.1080/13102818.2023.2226741
