About us
Research Professionals CRO is a Contract Research Organization (CRO) that manages clinical studies associated with the development of new pharmaceutical products and medical devices.
We are confident that we offer only the best clinical research teams, so that together with our sponsor customers, we can seamlessly find solutions to the clinical study challenges they may face. With exceptional local expertise, we are constantly challenging ourselves to exceed the quality and capabilities of leading global contract research organizations.
At Research Professionals CRO we believe that our service-driven approach to meeting market needs, leads to more efficient and less costly clinical research studies. Our objective is always to provide the highest quality clinical research study services that accelerate the advancement of sponsor medicinal products and medical devices.
Board
The Research Professionals Board is highly committed to establishing strategic alliances with industry partners and is also passionate about creating novel service opportunities in the field of clinical research.
Our dedicated board members bring with them substantial work experience gained from roles with pharmaceutical, biotechnology and device industry leaders or the service companies that serve them including: Allergan, Amgen, AstraZeneca, Biogen, Pfizer, Sanofi, Chiltern, DOCS and MDS Pharma Services.
György BARTA, PharmD - managing partner
György is a clinical research professional with 17 years industry experience in both CRO and pharma environments. He gradually developed from CRA through local/regional project manager to a full-service global study manager, managing phase II-III. global clinical trials. He is an expert in risk-based monitoring and experienced in leading cross-functional study teams, working in a multinational environment.
“Work hard. Have fun and make it happen.“
Rafael Nadal
Kálmán TÖRŐCSIK, MD - managing partner
Kálmán held a number of Clinical Research Associate and a Project Manager positions at drug research firms of various sizes. He has been involved in several technology development projects, e.g. working on a patient travel reimbursement system for clinical trials and designing a medical device for the intensive care.
“Research is to see what everybody else has seen, and to think what nobody else has thought.”
Albert Szent-Györgyi
Zoltán SÁGI, MD MSc - consultant
Zoltán is a respiratory physician and has an economist degree in marketing. He has broad experience in strategy and business planning, partnership development, investor search and deal-flow management. He managed intellectual property issues and pre-sales efforts in Asia, Europe, the Middle East and North America. He has also been working at the National Health Service in the United Kingdom.
“That which does not kill us, makes us stronger.”
Friedrich Wilhelm Nietzsche
Staff
At Research Professionals (RP-CRO), we pride ourselves on being a full-service contract research organization (CRO) dedicated to delivering high-quality, efficient clinical trial management. With a broad array of expertise, our team is composed of specialists across numerous therapeutic areas and phases of clinical research, making us uniquely positioned to guide your project to success.
The foundation of our success lies in our commitment to personalized service and streamlined communication. With a single point of contact assigned to each project, we simplify project management and maintain accountability throughout the entire clinical trial process. This not only speeds up decision-making but also ensures that any challenges are swiftly addressed and resolved. We aim to support your project at every step, from study design through patient recruitment, site selection, data management, and final regulatory submissions.
Our team is particularly adept in managing complex and specialized clinical trials. Whether it's Phase I exploratory studies or large-scale Phase III trials, our experience spans all phases of clinical research.
At RP-CRO, our goal is to be your trusted partner in clinical research, delivering results with precision and care. Whether you are embarking on a new clinical trial or looking to advance your drug development program, we are here to help you navigate the complexities of clinical research with confidence. Together, we will ensure that your study reaches its milestones, complies with regulatory standards, and delivers impactful results.
Our Growth
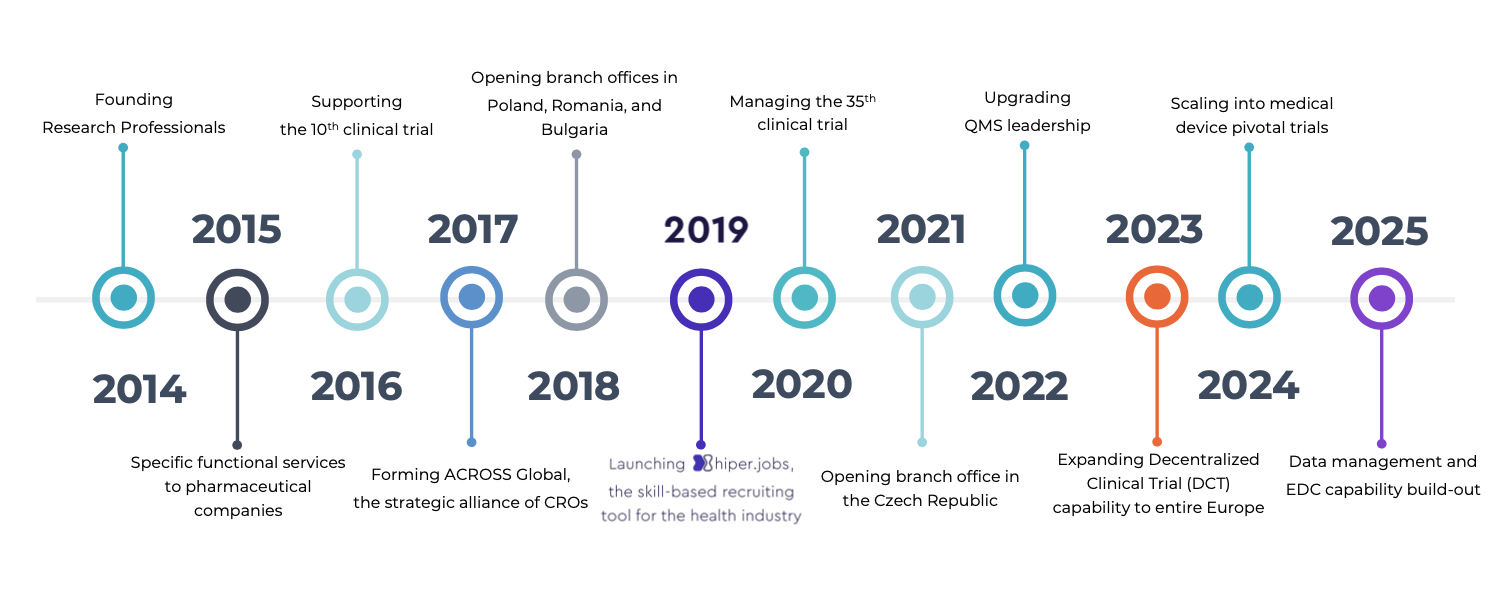
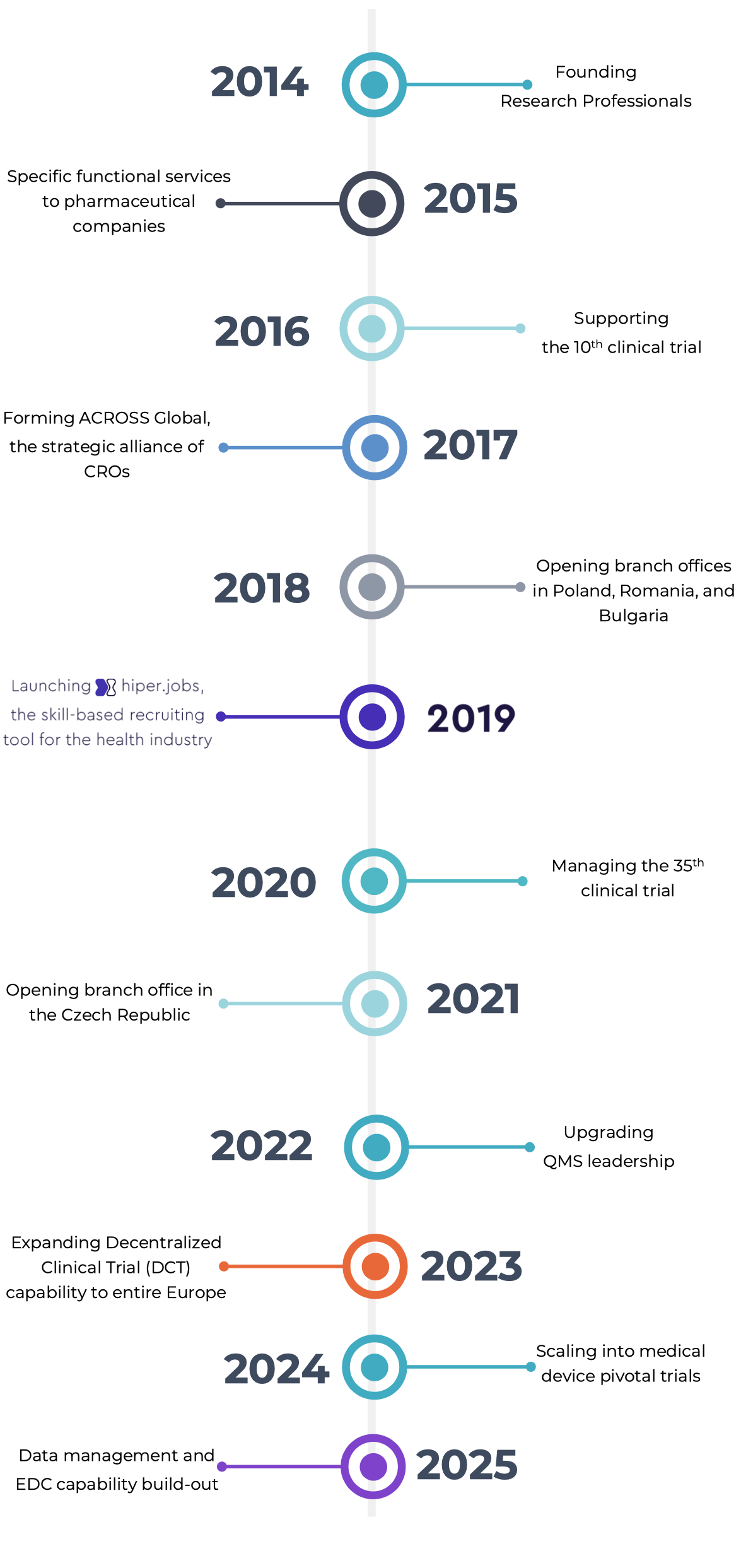
2014
Founded
Research Professionals Ltd
2015
Managing
1st home care study
2016
Monitoring
the 10th clinical trial
2017
Forming ACROSS Global,
a strategic alliance of CROs
2018
Opened branch offices in
Poland, Romania, and Bulgaria
2019
Developing HIPER, a skill-
based workforce platform for clinical research